Custom Medical Plastic Parts Technical Guide
Expert Analysis of Precision Manufacturing Processes and Material Selection

Dr. Zhang Ming
Medical Plastic Materials Expert | 18 Years of Industry Experience
Published on January 12, 2026
Product Overview
Medical plastic parts are essential components in modern medical devices, crafted from biocompatible plastics. They are widely used in various medical equipment from surgical tools to diagnostic devices, offering advantages such as sterility, durability, and cost-effectiveness.
Customized medical plastic parts can be personalized designed according to specific medical needs, ensuring precision, safety, and optimal performance in critical healthcare applications. Through precision manufacturing processes, products ranging from tiny implant components to large diagnostic equipment parts can be produced.
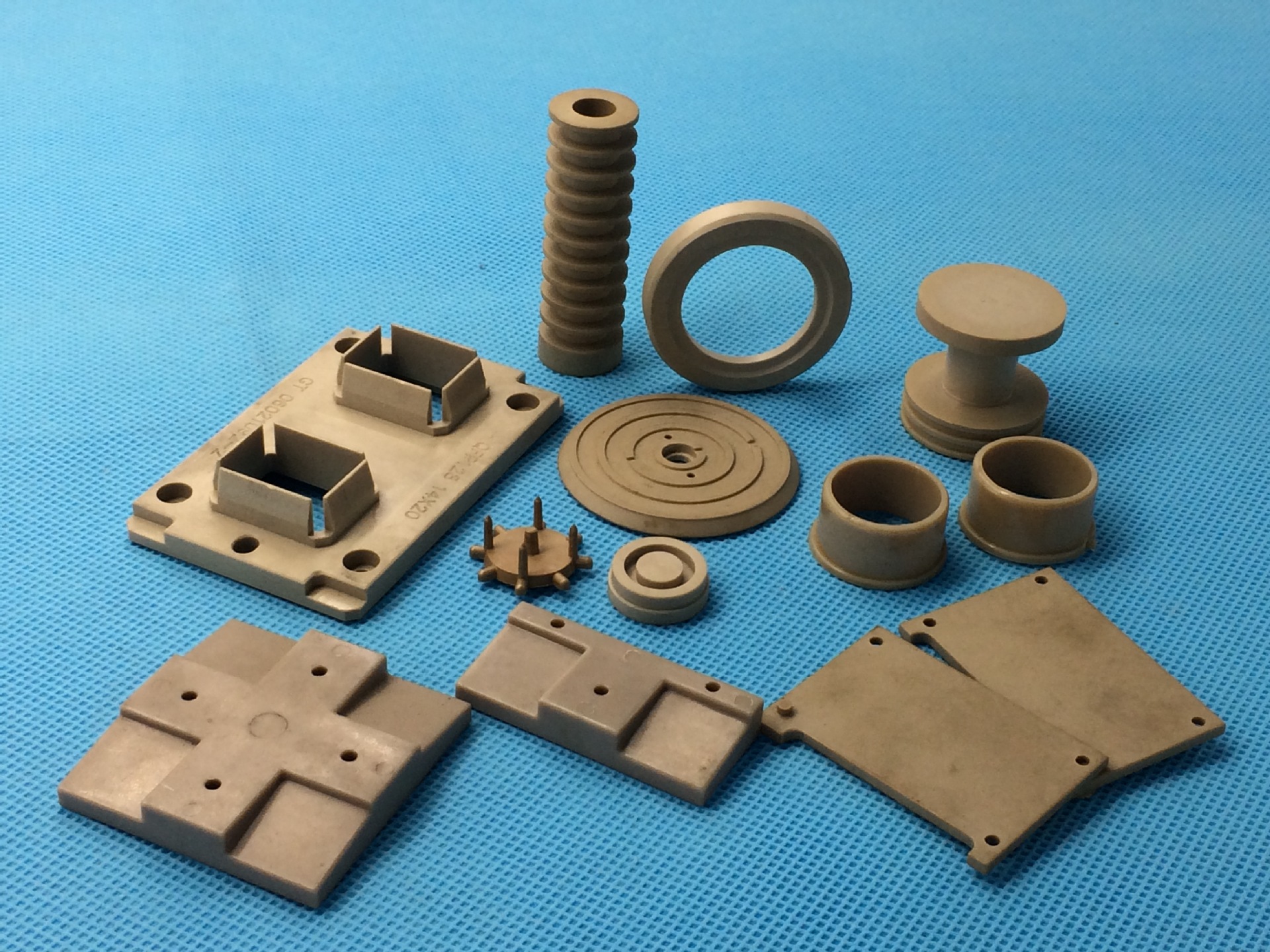
Core Features
Biocompatibility
Compliant with ISO 10993 standards, ensuring safety when in contact with human tissues and blood, non-toxic, non-sensitizing, and non-irritating.
Sterilizability
Able to withstand repeated sterilization without degradation, supporting multiple sterilization methods including gamma radiation, ethylene oxide, and steam sterilization.
High Precision
Strict tolerance control, typically achieving ±0.01mm, ensuring accurate functionality and perfect fit of medical devices.
Chemical Resistance
Able to resist corrosive disinfectants and bodily fluids, maintaining stable performance over the long term.
Material Selection Guide
Medical Grade Plastic Materials Comparison
| Material Type | Key Features | Applications | Sterilization Methods |
|---|---|---|---|
| PEEK | High strength, heat resistant, excellent biocompatibility | Orthopedic implants, spinal fusion cages | Gamma radiation, steam sterilization |
| PP | Low density, chemical resistant, cost-effective | Syringes, surgical instruments, packaging | Steam sterilization, ethylene oxide |
| PVC | Transparent, easy to disinfect, flexible when plasticized | Catheters, blood bags, oxygen masks | Ethylene oxide, gamma radiation |
| PE | Inert, flexible, easy to process | IV bags, catheters, laboratory equipment | Steam sterilization, ethylene oxide |
| ABS | Rigid, good dimensional stability, chemical resistant | Medical device housings, handles | Alcohol disinfection, ethylene oxide |
Material Selection Recommendations
Selecting the right medical plastic material requires comprehensive consideration of multiple factors:
- Application Environment: Whether long-term contact with human tissues is required
- Sterilization Requirements: Number of sterilization cycles and methods to be endured
- Mechanical Properties: Requirements for strength, toughness, wear resistance, etc.
- Cost Factors: Material costs and processing costs
- Regulatory Requirements: FDA, ISO certification requirements

Manufacturing Process Details
Injection Molding
Melts plastic resin at high temperature and injects it into mold cavities through an injection molding machine, then cools and solidifies to obtain molded parts.
- Suitable for high-volume, complex part production
- Precision可达±0.01mm~±0.05mm
- PEEK injection temperature needs 360-400℃
- Mold temperature needs to be maintained at 140-180℃
3D Printing Technology
Builds parts layer by layer from medical-grade filaments, suitable for small-batch, complex designs and personalized customization.
- FDM/FFF technology: heats filament to 380-400℃
- SLS technology: laser sinters powder materials
- Enables personalized customization and complex structures
- Suitable for patient-specific surgical guides and other applications
CNC Precision Machining
Precisely cuts plastic blocks to create detailed parts, suitable for rigid materials that are difficult to mold.
- Milling: processes planes, curved surfaces or complex contours
- Turning: processes cylindrical components
- Drilling/boring: creates through holes or stepped holes
- Precision可达±0.01mm

Thermoforming Process
Heats plastic sheets and forms them over molds, used for trays, enclosures, and other large, shallow parts.
- Suitable for producing large, thin-walled parts
- Lower cost, suitable for high-volume production
- Can produce complex-shaped enclosures
- Commonly used for medical device housings and trays

Technical Parameters and Test Data
Biocompatibility Test Results
ISO 10993 Standard Testing
* Experimental data is for reference only, actual results may vary depending on test conditions
Sterilization Test Data
Effect Comparison of Different Sterilization Methods
| Sterilization Method | Number of Cycles | Performance Retention Rate |
|---|---|---|
| Gamma Radiation | 50 cycles | 98.5% |
| Ethylene Oxide | 100 cycles | 97.2% |
| Steam Sterilization | 500 cycles | 96.8% |
| Vaporized Hydrogen Peroxide | 25 cycles | 99.1% |
* Data based on PEEK material testing, other materials may vary
Precision Tolerance Standards
General Precision
±0.1mm ~ ±0.2mm
Suitable for non-critical components
High Precision
±0.05mm ~ ±0.1mm
Suitable for general medical devices
Ultra-High Precision
±0.02mm ~ ±0.05mm
Suitable for implants and precision instruments
Extreme Precision
±0.01mm
Suitable for special requirement components
Quality Assurance and Certifications
ISO 13485 Certified
Our quality management system is certified to ISO 13485, ensuring consistent quality in medical device manufacturing.
Cleanroom Manufacturing
Class 8 cleanroom facilities ensure contamination-free production of critical medical components.
Advanced Testing
State-of-the-art testing equipment for dimensional accuracy, material properties, and biocompatibility.
Regulatory Compliance
Application Fields
Diagnostic Equipment
Housings, test tube racks, sensor components, requiring high precision and stability.
Surgical Tools
Handles, instrument housings, disposable scalpel guards, etc.
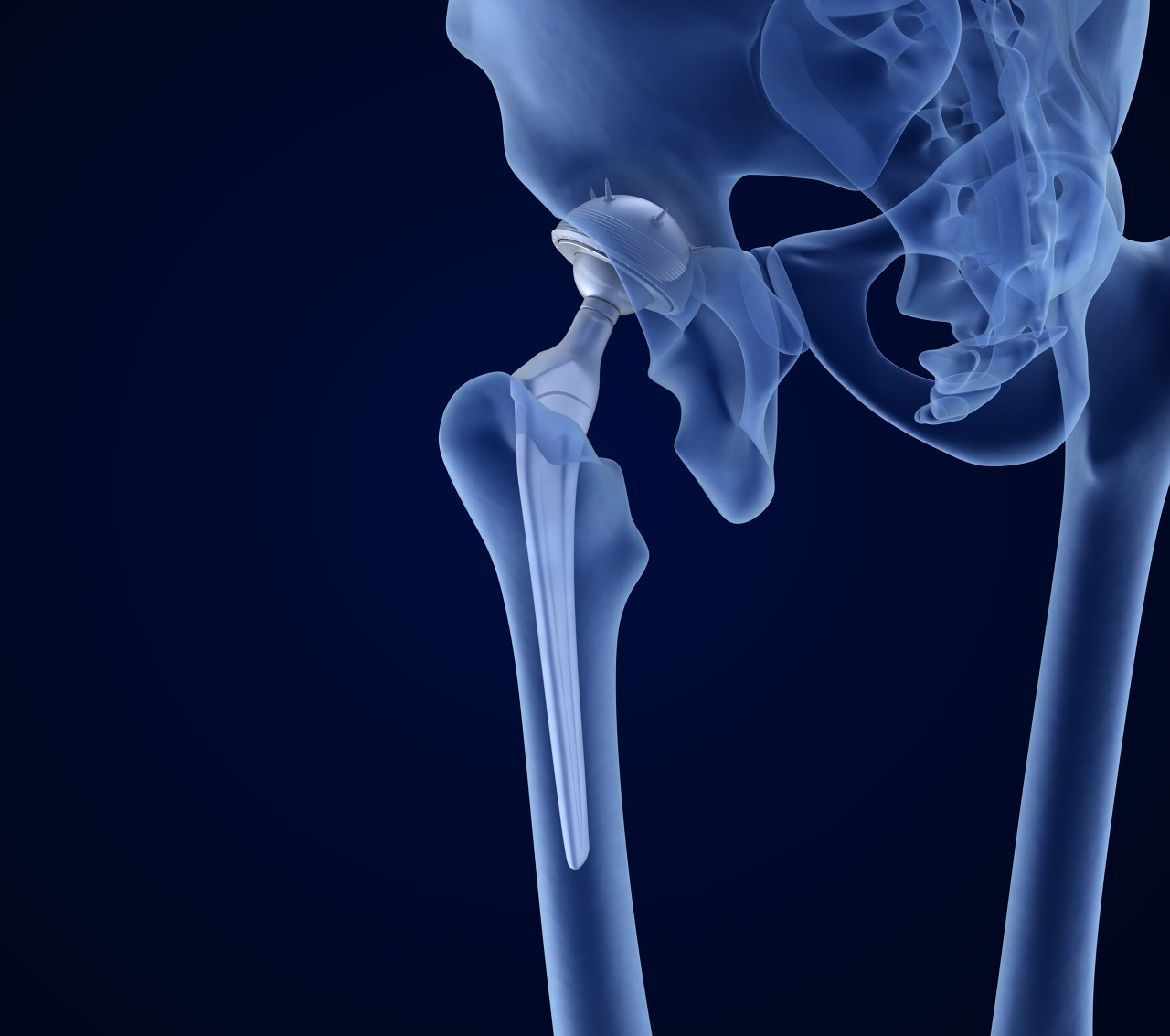
Implants
Orthopedic spacers, dental fixtures, typically using PEEK materials.
Patient Care
Syringes, IV components, wheelchair parts and other disposable and reusable products.
Our Expert Team

Dr. Zhang Ming
Chief Material Scientist
Ph.D. in Biomedical Engineering with 18 years of experience in medical plastic materials research and development.

Dr. Li Wei
Senior Process Engineer
Expert in precision manufacturing processes with 15 years of experience in medical device production.

Dr. Wang Hong
Quality Assurance Director
Specialized in regulatory compliance and quality management systems for medical devices.
Customization Service Process
Requirements Analysis
Detailed understanding of customer’s specific requirements, including dimensions, materials, biocompatibility, and regulatory compliance.
Design & Development
Design using CAD software, then 3D print or machine prototypes for testing and validation.
Material Selection
Choose appropriate medical-grade plastics based on application, such as PEEK for implants, PP for disposables.
Production Manufacturing
Mass production using injection molding, 3D printing or other technologies, ensuring quality and efficiency.
Quality Inspection
Strict dimensional inspection and performance testing to ensure products meet customer requirements and regulatory standards.
Packaging & Delivery
Sterile packaging ensures parts remain contamination-free until use, with timely product delivery.
Customization Advantages
- Personalized Design: Customized according to specific customer needs, meeting unique device design requirements
- High Precision Manufacturing: Ensures perfect fit, reduces contamination risks, improves device efficiency
- Regulatory Compliance: Meets FDA, ISO and other regulatory standards, ensuring product safety
- Rapid Response: Flexible production processes, able to quickly respond to market demand changes
- Cost Optimization: Optimizes costs based on volume and requirements, improving cost-effectiveness
Frequently Asked Questions
How to choose the right medical plastic material?
Selecting medical plastic materials requires considering multiple factors: application environment (whether contact with human tissues is required), sterilization requirements (number of cycles and methods to be endured), mechanical performance requirements (strength, toughness, wear resistance, etc.), cost factors, and regulatory requirements (FDA, ISO certifications). It is recommended to consult with professional material engineers to select the most appropriate material based on specific application scenarios.
What is the production lead time for medical plastic parts?
Production lead time depends on several factors: prototyping typically takes 1-2 weeks; mass production via injection molding takes 4-6 weeks; 3D printing can accelerate small-batch production. Specific timelines need to be determined based on product complexity, material selection, and production volume.
How is the cost of custom medical plastic parts calculated?
Costs mainly include: material costs (based on material type and usage), mold costs (required for injection molding), processing costs (based on process complexity and labor hours), quality inspection costs, and certification costs. Small-batch production costs may be higher, but unit costs decrease significantly as volume increases.
How to ensure biocompatibility of medical plastic parts?
Ensuring biocompatibility requires: selecting materials with biocompatibility certification, strictly controlling contamination during production, conducting necessary biocompatibility tests (such as ISO 10993 standard testing), and following relevant regulatory requirements. It is recommended to work with experienced medical device manufacturers who typically have comprehensive quality control systems.
How many times can medical plastic parts be resterilized?
The number of resterilization cycles depends on material type and sterilization method: high-performance plastics like PEEK can withstand over 500 steam sterilization cycles; PP materials can typically withstand 100-200 cycles; single-use materials are not recommended for resterilization. Specific numbers should reference data provided by material suppliers and be verified through actual testing.
Blog Conclusion
Custom medical plastic parts play a crucial role in modern medical devices. Through the detailed introduction in this article, we have learned about the characteristics of medical plastic materials, manufacturing processes, application fields, and customization service processes.
When selecting and using medical plastic parts, it is recommended to comprehensively consider material performance, processing technology, cost factors, etc., based on specific application scenarios and requirements, and choose the most suitable product solution. At the same time,Be sure to ensure products comply with relevant regulatory standards to ensure patient safety.