1. Technical Overview of Medical-Grade Injection Molding
Medical-grade plastic injection molding requires adherence to stringent regulatory standards (e.g., ISO 13485, FDA 21 CFR, and USP Class VI) to ensure biocompatibility, sterilizability, and traceability. These parts must withstand repeated sterilization cycles (autoclave, gamma radiation, or EtO) while maintaining dimensional stability and chemical resistance. Key applications include surgical instruments, implantable devices, drug delivery systems, and diagnostic equipment. Chinese suppliers specializing in medical molding integrate cleanroom production (ISO Class 7/8), validation protocols (IQ/OQ/PQ), and material certification to meet global healthcare demands. This article examines material selection, surface treatments, and validation workflows critical for medical device manufacturing.

2. Technical Documentation: Medical-Grade Plastic Materials Comparison
Medical plastics must balance biocompatibility, mechanical performance, and sterilization compatibility. Below is a comparison of common materials:
| Material | Biocompatibility | Max Sterilization Temp (°C) | Chemical Resistance | Cost per kg (USD) | Typical Applications |
|---|---|---|---|---|---|
| PEEK | ISO 10993 compliant | 250 (Autoclave) | Excellent | 80–120 | Spinal implants, surgical tools |
| PC | USP Class VI | 121 (EtO) | Moderate | 4.0–5.5 | IV connectors, transparent housings |
| PP | USP Class VI | 135 (Gamma) | High | 2.5–3.5 | Syringes, disposable labware |
| PEI (Ultem) | ISO 10993 | 180 (Steam) | Excellent | 15–25 | Endoscope components, sterilization trays |
| PSU | USP Class VI | 160 (Dry heat) | High | 10–18 | Dental instruments, fluid handling |
Engineer Recommendations:
- PEEK: Mandatory for long-term implantables and high-load surgical tools.
- PP: Cost-effective for single-use devices requiring gamma sterilization.
- PEI: Ideal for steam-autoclavable reusable instruments.
- Silicone (LSR): Use for seals, gaskets, or soft-touch components.
3. Customer Case Study: Custom Insulin Pump Housing
Client Pain Point: A diabetes care company needed a miniaturized, ISO 10993-compliant housing resistant to ethanol wiping but struggled with stress cracking and particulate contamination.
Technical Challenges:
- Achieving wall thickness <1.0 mm without warping.
- Ensuring biocompatibility for skin contact (cytotoxicity <0.5%).
Solution: - Material: Medical-grade PC/ABS blend (UL 94 V-0 flame retardant).
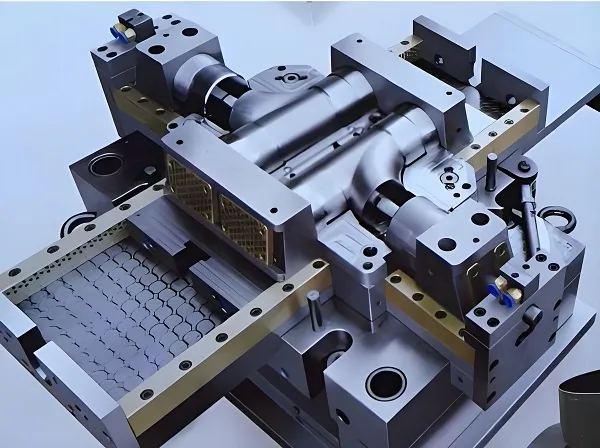
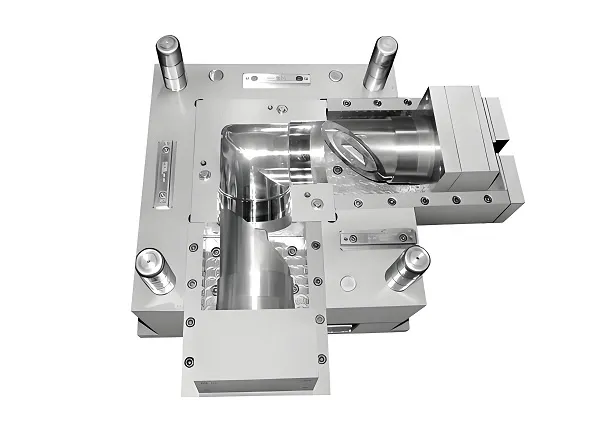
- Tooling: Stainless steel mold with nano-coated cavities to reduce friction.
- Process: Micromolding with vacuum degassing to eliminate voids.
Results: - Particulate count reduced to <5 particles/mL (meets USP <788>).
- Production yield increased from 82% to 98.5%.
4. Surface Treatments for Medical Components
| Process | Characteristics | Applications |
|---|---|---|
| Plasma Treatment | Enhances adhesion for bonding/coating | Catheters, biosensor surfaces |
| Autoclave-Ready Coating | Anti-microbial layer, withstands 100+ cycles | Surgical instruments, reusable devices |
| Electropolishing | Removes microburrs; improves cleanability | Implantable components, scalpels |
| Texture Etching | Non-slip grip; masks handling scratches | Handheld diagnostic tools |
5. Medical-Grade Customization Workflow
- Regulatory Review: Define biocompatibility, sterilization method, and FDA/EU MDR compliance.
- Material Certification: Verify resin purity (e.g., ISO 10993-5 cytotoxicity testing).
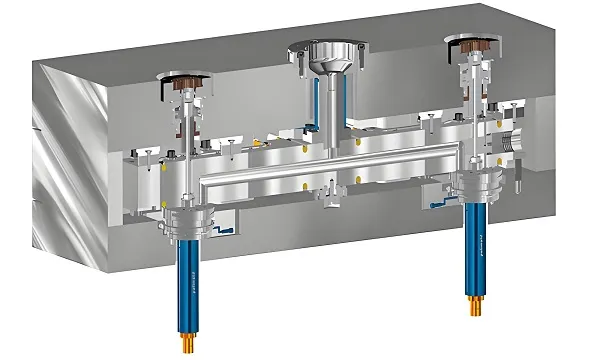
- Tooling Design: Hardened steel molds with EDM finishing for <0.1µm Ra surfaces.
- Cleanroom Molding: ISO Class 8 environment with particle monitoring.
- Post-Processing: Ultrasonic cleaning and packaging in sterile barrier systems.
6. FAQ: Medical Plastic Injection Molding
Q: What documentation is required for FDA approval?
A: Submit Material Certificates (USP/ISO), Biocompatibility Reports, and Process Validation Records (IQ/OQ/PQ).
Q: Can silicone (LSR) be overmolded onto rigid plastics?
A: Yes, using two-shot molding for seals or soft-grip features.
Q: How to validate sterilization compatibility?
A: Conduct accelerated aging tests per ISO 11607 and ASTM F1980.